There’s more than one way to propagate a plant. Growing from seeds, cuttings, or grafting are tried and true methods well-known in the annals of horticulture. Tissue culture, of more recent origin, is a method that can generate a large number of plantlets in a comparatively small space. Although more technically advanced, tissue culture methods can generate more genetically identical plants in a shorter time than is possible with vegetative propagation of mother plants by cuttings.
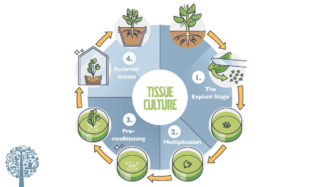
In plant tissue culture, as the name suggests, plant tissue is disinfected, cut up, and placed on a growth medium designed to encourage cell proliferation. Then the culture is induced to promote shoot growth. Once shoots appear, another treatment initiates root development. Rooted shoots are then removed from the tissue culture environment and transplanted into the soil or other typical medium. After a period of acclimation, the young plants are ready to survive independently. The process is usually divided into three or four stages. Although plant tissue culture procedures vary, the following gives a general overview of the technique depending on the lab.
Stage 1 – The Explant Stage
Begin with a piece of stem, leaf, meristematic tissue, or any other plant part. Exact protocols vary, but the material is excised from the plant and put through a disinfection sequence involving soaking and rinsing in some combination of isopropanol, bleach solution, and sterile water. The explants are placed on a medium containing a carbon source, essential nutrients, and one of the auxin plant hormones. The carbon source is often sucrose, and the hormone can be indole-3-butyric acid (IBA), 2,4-dichlorophenoxyacetic acid (2,4 D), or one of several other auxins. Explants will often initiate growth as an undifferentiated mass of cells called callus. Sometimes shoots will begin to form from the callus tissue. The synthetic hormone 2,4 D is especially good at promoting the growth of undifferentiated callus tissue.
Stage 2 – Multiplication
In stage II, the goal is to generate shoots by branching existing shoots or by new bud formation. The ratio of cytokinins to auxins is adjusted to encourage the initiation of new shoots from the callus tissue. Cytokinins promote cell division. Although a high auxin concentration is required to initiate and maintain callus growth in the explant stage, increasing the cytokinin to auxin ratio triggers shoot organogenesis in stage II. Any shoots that arise on the stage I medium are dissected and separated. Then they are replanted into a medium containing a low concentration of auxin plus the addition of kinetin, benzylaminopurine (BA), isopentyladenine (2iP) or other cytokinins. If no shoots form on the stage I medium, sub-samples of callus tissue are plated onto the stage II medium to induce shoot organogenesis. Sometimes only one or two shoots will form, and other times, a dozen or more may form, depending on the plant species and growth conditions.
During stage II, the real power of tissue culture can be realized. The multiplication step can be repeated until any desired number of shoots becomes available. Each multiplication cycle takes anywhere from one to several months, but it is possible to generate thousands of shoots in a small space relatively quickly.
Stage 3 – Preconditioning
One might also call this the “rooting” stage. Once healthy shoots are formed on the multiplication medium, they are excised and replanted into a medium lacking in cytokinin but once again providing auxin, which is essential for root organogenesis under these conditions. The auxin can be included in the medium, or each shoot can be dipped in rooting hormone before sticking it into the new medium. Once roots appear, the plants can grow until enough root mass exists to divide among the shoots.

Stage 4 – Acclimation
Once plantlets with a good shoot and good root development are established in vitro, it is time to get them out of the culture vessels and into the real world. This is an excellent time to be selective by only choosing plants with strong root and shoot development for transfer to the final stage. Often, wild and unruly plants result in the healthiest and most survivable transplants. Well-rooted plantlets are excised, divided, and transplanted into the soil in pots. The young plants are sensitive to light, water stress, and disease since they have led a sheltered life up to this point. The plants must be kept in a humid environment to avoid a high loss rate. We can do this by placing a small humidity dome made from a clear plastic cup or a plastic bag over each potted plant. Over a few weeks under fluorescent or LED illumination—no direct sun or high powered lights—the humidity chambers are gradually vented to allow a reduction in the humidity until the plants can survive on their own under normal growth conditions.
One key to success in plant tissue culture is the maintenance of axenic growth conditions. Axenic means “without a stranger”, meaning the only organisms allowed in the cultures are the plants and nothing else. The growth media used in tissue culture are perfect for supporting the growth of not only plants but also bacteria and fungi. Microbes will quickly destroy young tender plants if given the chance. For this reason, a laminar flow hood with a high-efficiency particulate air (HEPA) filter is recommended to push sterile air over the work zone during operations when the cultures are exposed. Even a non-filtered plexiglass glove box will decrease contamination by preventing particles from falling onto the plants and into the growth medium from above during processing. Lacking a flow hood, some success can be had by working quickly in a room with low airflow. Thoroughly clean work surfaces and equipment with 70% isopropanol and/or flame all tools and glass culture vessels. Technicians should, of course, wear gloves, wear a clean lab coat (no short sleeves), a hairnet, and a beard cover (if applicable) to reduce the chance of contamination as much as possible.
Expenses
So, how much does it cost to get into plant tissue culture? At a minimum, you’ll need a sterilizer/pressure cooker, glass or plastic ware (flasks, etc.), measuring equipment (electronic or triple beam balance, graduated cylinders), culture media ingredients and hormones, and a culture shelf with lights. An instant pot and baby food jars can get you started. Unless you already have basic laboratory equipment, expect to spend anywhere from $300-$500 upfront. Like most technical lab procedures involving living things, it takes some practice and experience to succeed. Keep accurate records in a notebook, and don’t give up until it reliably works. You’ll never forget your first in vitro plantlet.